Introduction
The AQUAMAT trial is the largest hospital-based clinical trial for severe malaria to date. It was carried out between 2005 and 2010 in 11 sites in 9 countries across sub-Saharan Africa and recruited children with severe malaria who were randomized to receive either parenteral quinine or artesunate. Parenteral treatment was completed with a full course of oral artemisinin combination therapy. For an overview of the sites used during the study, refer to the table (right).
The primary outcome measure was in-hospital mortality, with neurological disability assessed at 28 days as an important secondary outcome.
Results of the AQUAMAT trial were pivotal in updating WHO Standard Treatment Guidelines to include injectable artesunate as the standard of treatment
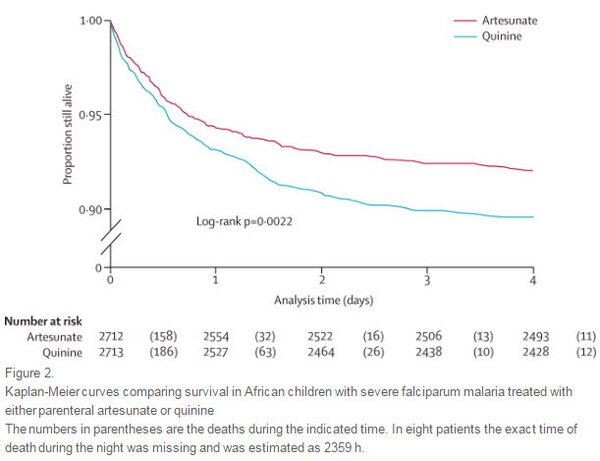
Difference in mortality rates between patients given injectable artesunate and injectable quinine.
Conclusions of study
Artesunate substantially reduces the overall mortality of African children diagnosed with severe malaria (8.5% with artesunate compared to 10.9% with quinine)
The incidence of confirmed persistent severe sequelae after severe malaria was low
Artesunate has a much broader stage-specificity of action than quinine. The artemisinins kill circulating ring-stage parasites before they can mature, reducing sequestration of erythrocytes, preventing potentially lethal microvascular obstruction
The benefits of parenteral artesunate compared with quinine were greater than the benefits of intramuscular artemether reported in previous trials
The benefits of artesunate were greater in patients from southeast Asia than in African children (though this cannot be said with certainty)
Parenteral artesunate is simple to administer, is well tolerated, and reduces mortality substantially compared with quinine.
No serious adverse effects were identified
Artesunate should become the treatment of choice for severe malaria for children and adults worldwide