South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT)
The SEAQUAMAT trial was a multicentre, open-label, randomised comparison of parenteral artesunate (Inj AS) and parenteral quinine (Inj Q) in patients with severe P. falciparum over the age of two. The study was carried out between June 2003 and May 2005 in Bangladesh, Myanmar (Burma), India, and Indonesia.
Based on previous studies that suggested Inj AS as more beneficial over artemether and positive results from an initial pilot comparing quinine with artesunate, the aim of the study was to establish conclusively which, of parenteral artesunate or parenteral quinine, is the more effective drug for the treatment of severe malaria.
The study was stopped by DSMC after 1461 patients were enrolled and had received either Inj AS (730 assigned) or Inj Q (731 assigned). There were 202 children (younger than age 15 years) in the study of whom 89 were aged younger than 6 years.
Conclusions of the study
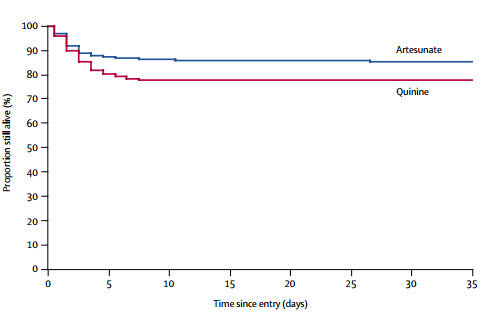
- Mortality in artesunate recipients was 15% (107 of 730) compared with 22% (164 of 731) in quinine recipients
- An absolute reduction of 34.7% in mortality for patients treated with Inj AS
- Treatment with artesunate was well tolerated, whereas quinine was associated with hypoglycaemia
- The conclusion of the study determined that Inj AS should become the treatment of choice for severe P. falciparum malaria cases in adults.
- This finding suggests that the superiority of artesunate over quinine in severe malaria might be less obvious in African children, the AQUAMAT study was designed to provide a definitive answer to this question.